Introduction: AML is an aggressive disease with poor prognosis that predominantly affects older adults. Due to advanced age and associated comorbidities, many patients are not fit for intensive induction chemotherapy. Monotherapy with HMAs such as azacitidine (AZA) or decitabine (DEC) is often still considered as standard of care for these patients, despite mixed evidence from studies regarding the benefit of HMAs alone (Duchmann & Itzykson. Int J Hematol 2019). The aim of the current study is to evaluate patient characteristics, treatment patterns and outcomes of patients with AML treated with HMA monotherapy as first line (1L) in clinical practice in the US.
Methods: This is a retrospective observational study of the Flatiron Health database; a nationwide, longitudinal, demographically and geographically diverse database representing more than 2.4 million patients with cancer in the US. The database contains de-identified data derived from electronic health records from over 280 cancer clinics, which are predominantly community oncology practices. Patients ≥18 years, diagnosed with AML between 1/1/2014 and 3/30/2020 (excluding acute promyelocytic leukemia and clinical trial enrollment), and who received HMAs as 1L treatment ≤30 days from AML diagnosis were evaluated. Descriptive analyses were conducted on patient characteristics and treatment patterns. Kaplan-Meier analyses were used to estimate time to last administration (TTLA; from initiation to last observed administration before death, end of follow-up or a gap of 60 days) and median overall survival (OS).
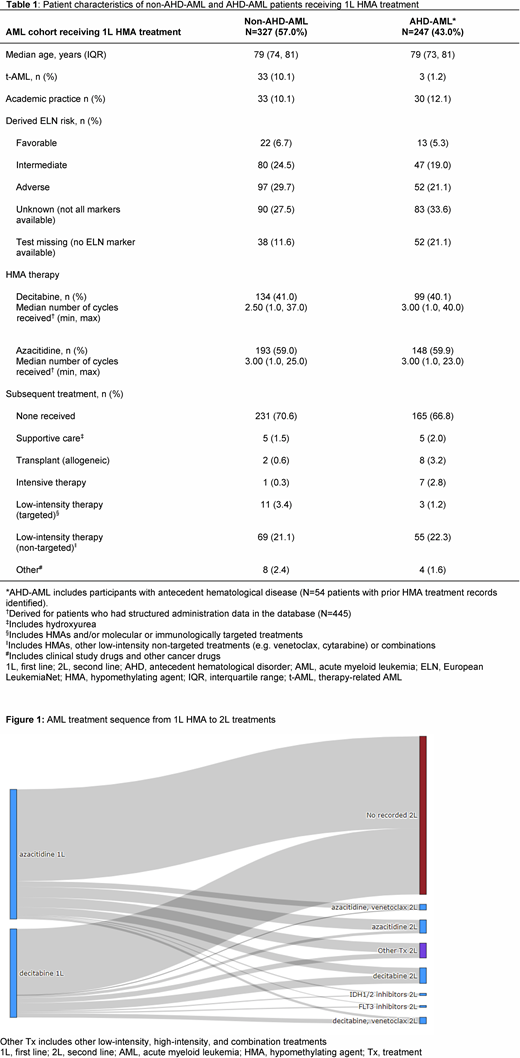
Results: A total of 2589 patients with an AML diagnosis were included for analysis, where 574 (22%) were treated with 1L HMAs (AZA: n=341 [59%]; DEC: n=233 [41%]). The median age of 1L HMA patients was 79 years with 63% male. Most patients were treated in the community setting (n=511 [89%]; median age: 79 years); those treated in academic centers were slightly younger (n=63 [11%]; median age: 77 years). Characteristics for non-antecedent hematological disorder (AHD)-AML (n=327) and AHD-AML (n=247) patients are presented in Table 1. Median TTLA with 1L HMA was 77 days with a median of 3 cycles of both AZA and DEC. Of the 168 patients who received second-line (2L) therapy, 82% (n=138) received another low-intensity therapy or combination (of which only 14 received targeted therapies) (Figure 1). Overall, 44% of 1L HMA patients (n=254) had evidence of molecular testing before 1L treatment initiation (this was more common in later years). Of the 228 patients tested for FLT3, 30 (13%) were FLT3 positive; 7 (23%) FLT3-positive patients were treated with 2L or third-line (3L) FLT3-targeted therapies (gilteritinib, midostaurin or sorafenib). Of the 152 patients tested for IDH1/2, 35 (23%) were IDH1/2 positive; 5 (14%) IDH1/2-positive patients were treated with 2L or 3L targeted agents (enasidenib or ivosidenib). A median OS of 6.3 months (95% CI: 5.5-7.5) was observed in the overall 1L HMA cohort. Median OS in 1L HMA patients did not differ with respect to different types of AML (non-AHD-AML: 6.6 [95% CI: 5.5-7.9] months; AHD-AML: 6.0 [95% CI: 4.8-7.5] months, p=0.34) or practice setting (community: 6.0 [95% CI: 5.3-7.0] months; academic: 8.3 [95% CI: 6.9-13.3] months, p=0.14). One-year OS was 31.4% and 30.1% for non-AHD-AML and AHD-AML patients, respectively. Patients treated in the community setting had numerically lower 1-year OS (29.7% [95% CI: 25.8-34.3]) than those treated in the academic setting (39.5% [95% CI: 28.6-54.6]), which reflects the higher rates of 2L treatment in academic practice, though this analysis is unadjusted.
Conclusions: This new database enabled a detailed analysis of 1L HMA-treated patients with newly diagnosed AML in routine clinical practice predominantly in the community setting. 1L HMA patients have poor survival outcomes (median OS 6.3 months) which are comparable to other real-world data from SEER-Medicare (Zeidan et al. Blood Adv 2020; median OS 7-8 months; median age: 77 years); but shorter than the median OS of 9-10 months observed in 1L HMA-treated AML patients in clinical trials (DiNardo et al. EHA 2020). Limitations of the study included limited conduct of bone marrow biopsies for response and lack of transfusion data. The observed survival outcomes highlight the importance of further treatment advances to address the unmet need in older patients with AML ineligible for intensive induction chemotherapy.
Pardee:Rafael: Research Funding; Celgene: Consultancy, Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Pharmacyclics: Speakers Bureau; Rafael Pharmaceuticals: Consultancy; BMS: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy; Genentech, Inc.: Consultancy; Karyopharm: Research Funding. Oschwald:Roche Products Limited: Current Employment. Ma:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Xu:F. Hoffmann-La Roche Ltd: Current Employment, Other: All authors received support for third party writing assistance, furnished by Scott Battle, PhD, provided by F. Hoffmann-La Roche, Basel, Switzerland.. Montez:F. Hoffmann-La Roche: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Ramsingh:Genentech, Inc.: Current Employment; NEKTAR: Current equity holder in publicly-traded company; Exelixis: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months; F. Hoffmann-La Roche: Current equity holder in publicly-traded company. Hong:Genentech, Inc.: Current Employment; F. Hoffmann-La Roche: Current equity holder in publicly-traded company. Choi:AbbVie: Current Employment, Current equity holder in publicly-traded company. Flahavan:Roche Products Ltd.: Current Employment; F. Hoffmann-La Roche: Current equity holder in publicly-traded company.
Discussion will include the use of decitabine for the treatment of AML.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal